Jack R. answered • 07/13/20
Experienced ACT Prep/Organic Chemistry tutor
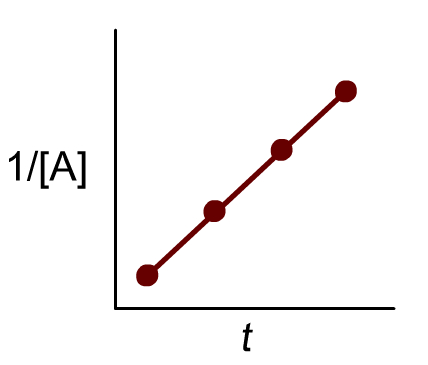
This is a second order reaction, as the graph of 1/[Concentration] vs time is linear. Thus, the units of k are M^-1s^-1. When you plot the data points =given according to this line, you get a slope of roughly 0.0133; this is the rate constant.