J.R. S. answered • 03/23/24
Ph.D. University Professor with 10+ years Tutoring Experience
For this question, we'll want to use the following relationship:
∆G = ∆Gº + RT ln Q
N2(g) + 3H2(g) <==> 2NH3(g)
Q = (NH3)2 / (N2)(H2)3
Q = (0.0100)2 / (0.603)(1.00)3
Q = 1.66x10-4 and since this is LESS than Kp, the reaction is NOT at equilibrium and will proceed to the right, i.e. toward the products.
Solving for ∆Grxn, we have...
∆G = 41,900 J + (8.314 J/Kmol)(650K) ln 1.66x10-4
∆G = -5135 J = -5.13 kJ



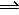 2NH3(
2NH3(


