J.R. S. answered • 03/21/24
Ph.D. University Professor with 10+ years Tutoring Experience
NH4OCONH2(s) <==> 2NH3(g) + CO2(g)
xs....................................0..............0..............Initial
......................................+2x...........+x............Change
........................................2x.............x............Equilibrium
Kp = [NH3]2[CO2]
0.00432 = (2x)2(x) = 4x3
x3 = 0.00108
x = 0.103 atm
Total pressure = 2x + x = (2 * 0.103) + 0.103
Total pressure = 0.309 atm



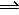 2NH3(
2NH3(


