J.R. S. answered • 03/21/24
Ph.D. University Professor with 10+ years Tutoring Experience
3H2(g) + N2 (g)<==> 2NH3(g)
0500.....0.900.............0...........Initial
-3x.........-x................+2x.........Change
0.5-3x....0.9-x.............2x.........Equilibrium
Kp = (NH3)2 / (H2)3(N2)
4.30x10-4 = (2x)2 / (0.5 - 3x)(0.9 - x)
4.30x10-4 = 4x2 / 3x2 - 3.2x + 0.45
Use quadratic to solve...
x = 0.00680 atm
Equilibrium partial pressure of NH3 = 2x = 2 x 0.00680 atm = 0.0136 atm



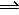 2NH3(
2NH3(


