J.R. S. answered • 03/01/24
Ph.D. University Professor with 10+ years Tutoring Experience
PCl5(g)
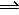 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
0.5…………..0.5050……0……I
-x……………,.+x……….+x……C
0.5-x……….0.5050+x……x…..E
Kp = (PCl3)(Cl2) / (PCl5)
25.1 = (0.5050+x)(x) / 0.5-x
Solve for x and then fill in the E (equilibrium) line of th ICE table to obtain partial pressures of all species present.





