J.R. S. answered • 03/02/24
Ph.D. University Professor with 10+ years Tutoring Experience
H2O(g) + Cl2O(g)
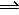 2HOCl(g)
2HOCl(g)
0.003923…0.003923…….0…….I
-x……………….-x………..+2x….C
0.003923-x…0.003923….2x……E
Kc = 0.090 = [HOCl]2 / [H2O][Cl2O]
0.090 = (2x)2 / (0.003923-x)2 and assume x is small, we simplify to
0.090 = (2x)2 / (0.003923)2
0.090 = 4x2 / 1.55x10-5
4x2 = 1.39x10-6
x = 5.88x10-4 M
Unfortunately, this value is NOT insignificant relative to 0.003923 and so it cannot be ignored and the calculation cannot be simplified in the above manner.
You will have to solve for x using the quadratic formula or by using some other simplifying assumption.
0.090 = (2x)2 / (0.003923)2
0.30 = 2x / 0.003923
x = 0.000588 M
[H20] = 0.003923 - 0.000588 = 0.003335 M
[Cl2O] = 0.003335 M
[HClO] = 2 x 0.003335 = 0.00667 M





