J.R. S. answered • 10/21/23
Ph.D. University Professor with 10+ years Tutoring Experience
3H2(g) + N2(g) <==> 2NH3(g)
0.500....0.900..............0.............Initial
-3x..........-x...............+2x...........Change
0.5-3x....0.9-x...........2x............Equilibrium
Kp = (NH3)2 / (H2)3(N2)
4.3x10-4 = (2x)2 / (0.5-3x)3(0.9-x)
Since Kp is small (10-4) we can assume x is small and ignore it in the denominator, simplifying to...
4.3x10-4 = (2x)2 / (0.5)3(0.9)
4.3x10-4 = 4x2 / 0.1125
4x2 = 4.84x10-5
x2 = 1.21x10-5
x = 3.48x10-3 (note: this is small compared to 0.5 and 0.9 so above assumption was valid)
Equilibrium partial pressure of NH3 = 2x
Equilibrium partial pressure of NH3 = 6.96x10-3 atm



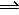 2NH3(
2NH3(


