J.R. S. answered • 08/24/23
Ph.D. University Professor with 10+ years Tutoring Experience
H2(g) + Cl2(g) <==> 2HCl(g) .. K = 5.08x10-9
2HCl(g) <==> H2(g) + Cl2(g) .. K = 1 / 5.08x10-9 = 1.97x108
The equilibrium constant for the reverse reaction (K')will be the reciprocal of the original K (1/K)


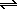 2HCl(g)
2HCl(g)


