J.R. S. answered • 08/24/23
Ph.D. University Professor with 10+ years Tutoring Experience
N2(g) + O2(g) 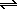 2NO(g) .. K = 2.51x1012
2NO(g) .. K = 2.51x1012
Because the equilibrium constant (K) is so large (so much greater than 1), we know that the reaction will lie very far to the right. That is to say, we know that there will be very little of the N2 and O2 left and the amount of NO will be very large. So option A would be correct.
If you want to actually do the calculations, you can set up an ICE table as follows:
N2(g) + O2(g) 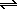 2NO(g)
2NO(g)
0..........0...............1.2.........Initial
+x........+x.............-2x.........Change
x...........x..............1.2-2x....Equilibrium
The K for the reverse reaction is 1 / 2.51x1012 = 3.98x10-13
3.98x10-13 = x2 / (1.2-x)2 and ignore the 2x since K is so small
3.98x10-13 = x2 / (1.2)2
x = 7.6x10-7 moles of N2 and O2 formed
So @ equilibrium we can predict the following concentrations:
[N2] = 7.6x10-7 moles / 44 L = 1.73x10-8 M
[O2] = 7.6x10-7 moles / 44 L = 1.73x10-8 M
[NO] = ~1.2 moles / 44 L = ~0.027 M





