J.R. S. answered • 12/03/22
Ph.D. University Professor with 10+ years Tutoring Experience
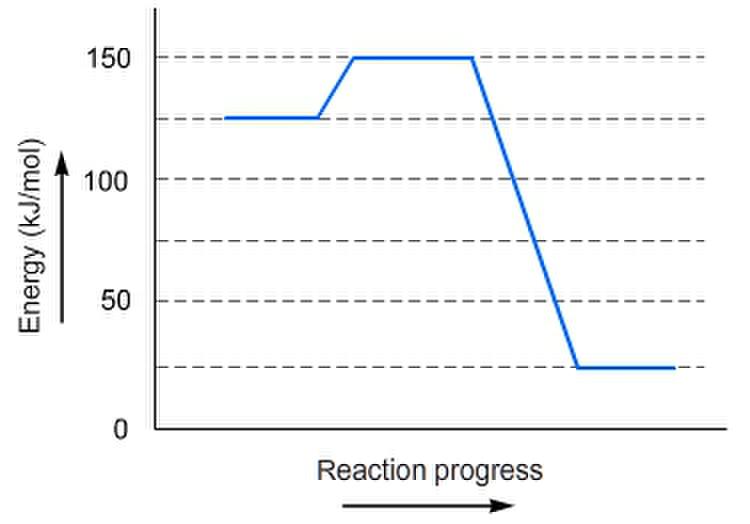
The activation energy is 25 kJ/mole. It's the difference between the 125 and 150 on the y-axis.
The reaction is EXOTHERMIC because the final energy of the products (25 kJ/mol) is less than that of the reactants (125 kJ/mol)