J.R. S. answered • 03/08/22
Ph.D. University Professor with 10+ years Tutoring Experience
IO- + H2O 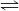 HIO + OH-
HIO + OH-
Kb = [HIO][OH-] / [ IO-]
0.00044 = (x)(x) / 0.713 - x and assume x is small relative to 0.713
x2 = 0.0003137
x = 0.0177 (which is only ~2.5% of 0.713 so above assumption was valid)
[OH-] = 0.0177 M
pOH = -log 0.0177 = 1.75
pH = 14 - 1.75
pH = 12.2
[H+] = 1x10-12.2 = 6.31x10-13 M (excluding the H+ from autolysis of H2O)





