J.R. S. answered • 04/07/21
Ph.D. University Professor with 10+ years Tutoring Experience
Full electron configuration would be 1s2 2s2 2p1
The name of the element would be boron (B) because there are 5 electrons and B has an atomic number = 5.
Alex R.
asked • 04/07/21A pictorial representation of an electronic configuration is shown.
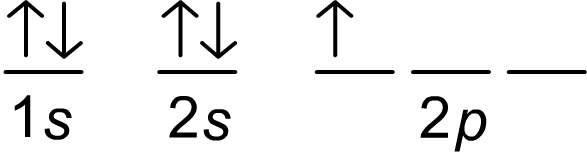
Give the full electron configuration AND NAME . Do not use the noble gas abbreviation.
J.R. S. answered • 04/07/21
Ph.D. University Professor with 10+ years Tutoring Experience
Full electron configuration would be 1s2 2s2 2p1
The name of the element would be boron (B) because there are 5 electrons and B has an atomic number = 5.
Get a free answer to a quick problem.
Most questions answered within 4 hours.
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.