J.R. S. answered • 01/11/21
Ph.D. University Professor with 10+ years Tutoring Experience
Use the Ideal Gas Law
PV = nRT
P = pressure in atmospheres
V = volume in liters = 3.2 L
n = moles = 0.200 moles
R = gas law = 0.0821 Latm/Kmol
T = temperature in Kelvin = 325K
Solve for P:
P = nRT/V = (0.200)(0.0821)(325) / 3.2 L
P = 1.67 atm (3 sig. figs.)




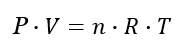



J.R. S.
01/11/21