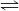J.R. S. answered • 10/01/20
Ph.D. University Professor with 10+ years Tutoring Experience
(1). Simply look at the coefficients in the balanced equation. The coefficient for HCl is 2 and that for K2SO3 is 1, so the mole ratio is 2 : 1
(2). Find moles of HCl and then since the mol ratio of HCl : K2SO3 is 2:1 (as determined above), we can find moles K2SO3 and then grams of K2SO3.
moles HCl = 26.5 ml x 1 L/1000 ml x 3.0205 mol/L = 0.08800 moles HCl
moles K2SO3 = 0.0800 mol HCl x 1 mol K2SO3 / 2 mol HCl = 0.0400 moles K2SO3 needed
mass K2SO3 needed = 0.0400 mol K2SO3 x 158 g/mol = 6.32 g K2SO3 needed (3 sig. figs.)