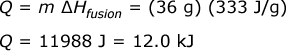J.R. S. answered • 01/09/20
Ph.D. University Professor with 10+ years Tutoring Experience
Since ∆Hfusion is used, and there is no ∆T (i.e. change in temperature), this shows that the process is a phase change. And the phase change would be (a) ice melting because the value is positive meaning heat is added to the system. If negative, the process would be "freezing".