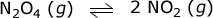J.R. S. answered • 01/09/20
Ph.D. University Professor with 10+ years Tutoring Experience
While the answer given by Moses I. is correct, the explanation is not. The left side of the equations does NOT have more moles of particles. The right side does. The right side has 2 moles of gas and the right side has only 1 mole of gas. According to Le Chatelier's Principle, any stress put on a system at equilibrium will be accommodated by a change to relieve that change.
If you decrease the pressure, since the right side has more moles, the equilibrium will shift to the right (toward products) in an effort to counteract that change.