Sebastien B. answered • 04/18/24
Tutor
New to Wyzant
Math, Physics, Chemistry - Experienced Credentialed Tutor/Teacher.
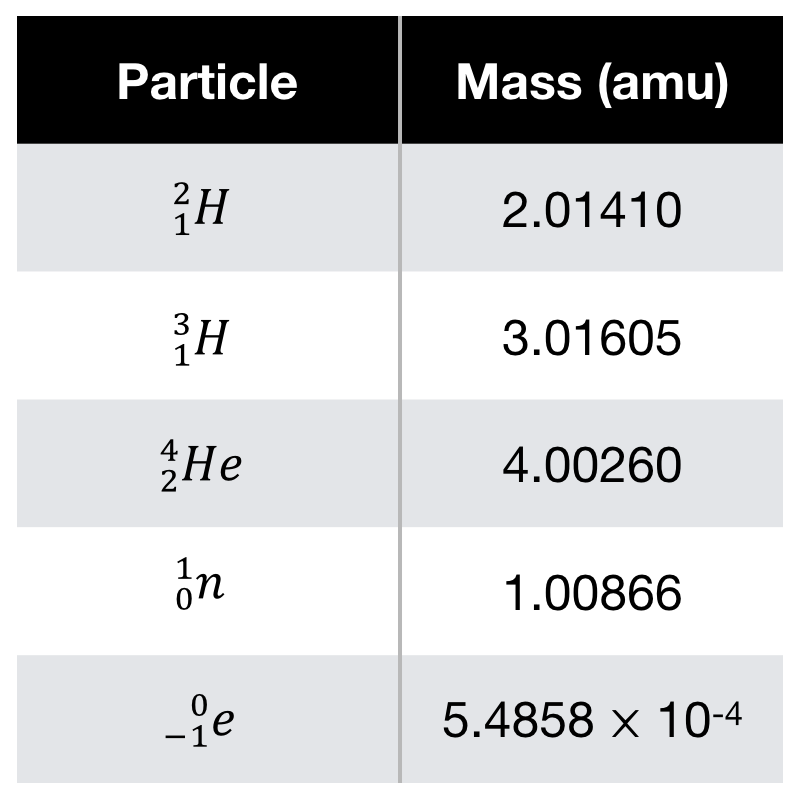
Δm = 4.00260+1.00866-3.01605-2.01410 = -0.01889 amu.
So, for 1 nucleus He, we have : E = IΔmI.C2 = 2.82x10-12 J (with C = 799,792,458 m/s).
So : E = 2.82x10-15 kJ for 1 nucleus of He.