J.R. S. answered • 10/21/23
Ph.D. University Professor with 10+ years Tutoring Experience
CO2(g) + C(s) <==> 2CO(g)
1.32......................... 4.85.............Initial
Q = (CO)2 / CO2) = (4.85)2 / 1.32 = 17.8
Compared to Kp (1.50), reaction is far to the right, so reaction will proceed to the left.
CO2(g) + C(s) <==> 2CO(g)
1.32......................... 4.85.............Initial
+x............................-2x................Change
1.32+x....................4.85-2x..........Equilibrium
Kp = (CO)2 / CO2)
1.50 = (4.85-2x)2 / (1.32+x)
1.50x + 1.98 = 4x2 - 19.4x + 23.5
4x2 - 20.9x + 21.52 = 0
x = 1.41
(A) Partial pressure CO @ equilibrium = 4.85 - 2.82 = 2.03 atm
(B) Partial pressure CO2 @ equilibrium = 1.32 + 1.41 = 2.73 atm



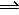 2CO(
2CO(


