Ajinkya J. answered • 10/10/22
Harvard UG Educated Math and Science Tutor. Online and In-Person.
The problem is based on the concept of limiting reagent. In a reaction if X and Y are the reactants and out of them Y is consumed completely and X is still left in the solution, then Y is the limiting reagent.
Limiting reagent is the reactant which gets completely used up in a chemical reaction.
The balanced chemical equation is the one with lowest whole number coefficients and same number of atoms on both sides of the equation.
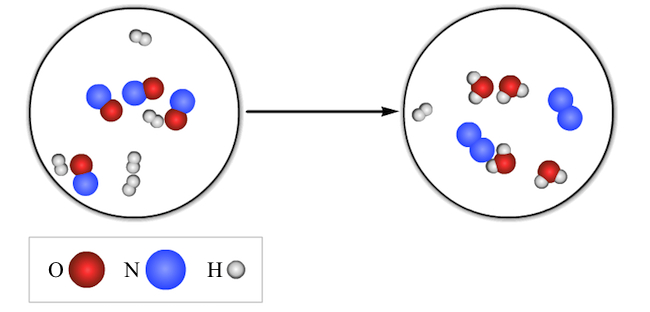
From the figure, it is concluded that the molecules that are present on the reactant side are 5 molecules of 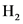 and 4 molecules of NO. The molecules that are present on the product side are 1 molecule of
and 4 molecules of NO. The molecules that are present on the product side are 1 molecule of 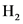 , 2 molecules of
, 2 molecules of 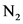 and 4 molecules of
and 4 molecules of 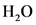 .
.
Hence the reaction becomes
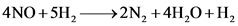
The molecules are counted from the figure and then on the basis of them, the chemical equation is written.
Here, all the NO molecules are consumed while 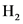 is still left in the reaction.
is still left in the reaction.
So, the limiting reagent is NO.
A limiting reagent is a reactant that gets completely used up in a chemical reaction. Here, NO is the limiting reagent because, after the reaction, no NO is left while 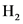 is still left in the solution.
is still left in the solution.
Now, the chemical equation is as follows
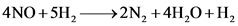
Balancing chemical equation, we get
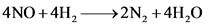
The balanced chemical equation with the lowest whole number coefficients will be

The balanced chemical equation is the one with the lowest whole number coefficients and the same number of atoms on both sides of the equation.
Answer:
The chemical formula of limiting reagent is NO