Ci = kHPi where C is the concentration of a gas dissolved in water and P is its partial pressure.
C = (7 x 10-4 mole/l-atm)(.53)(1.0 atm) because Pi = (ni/nT) PT or xiPT where x is the mole fraction.
Teagan L.
asked • 02/14/22If a gaseous sample contains 53% N2 by volume, what is the solubility of N2 in water at 25°C and 1.0 atm (kH in H2O at 25°C = 7.0  10-4 mol/L
10-4 mol/L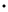 atm)?
atm)?
Ci = kHPi where C is the concentration of a gas dissolved in water and P is its partial pressure.
C = (7 x 10-4 mole/l-atm)(.53)(1.0 atm) because Pi = (ni/nT) PT or xiPT where x is the mole fraction.
Get a free answer to a quick problem.
Most questions answered within 4 hours.
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.