
Cheison P. answered • 09/28/20
Experienced Math, Physics and Chemistry tutor
Given
Weight of hydrated CaSO4.x H2O = W1 = 0.4813 g
Weight of anhydrous CaSo4 = W2 = 0.3750 g
Weight of H2O that was present as water of hydration is the difference between W1 & W2
W1 - W2 = 0.1063 g
CaSO4.x H2O 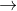 CaSO4 + x H2O 0.4812 g 0.3750 g 0.1063g
CaSO4 + x H2O 0.4812 g 0.3750 g 0.1063g
Molar Mass of anhydrous CaSO4 = 136.14 g/mol
Molar mass of H2O = 18.0153 g/ mol
So, convert that to moles, CaSO4 = Given mass / molar mass
= 0.3750 / 136.14 = 0.002755 mol
Number of moles of H2O = Given mass / molar mass
= 0.1063 / 18.0153 = 0.0059005 mol
So,
x = 0.0059005 / 0.002755
CaSO4 . 2 H2O

Cheison P.
Anytime09/28/20





Ooyeon O.
Thank you for your detailed explanation.09/28/20