
Monica K.
asked • 03/04/21How do I solve this
How long will it take before twenty percent of our 1,000-gram sample of uranium-235 has decayed? [See Section 6.6 Example 13]
The decay equation is 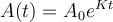 , where t is the time for the decay, and K is the characteristic of the material. Suppose T is the time it takes for half of the unstable material in a sample of a radioactive substance to decay, called its half-life. Prove that
, where t is the time for the decay, and K is the characteristic of the material. Suppose T is the time it takes for half of the unstable material in a sample of a radioactive substance to decay, called its half-life. Prove that 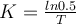 . What is K for the uranium-235? Show the steps of your reasoning.
. What is K for the uranium-235? Show the steps of your reasoning.
1 Expert Answer

Mark M. answered • 03/04/21
Mathematics Teacher - NCLB Highly Qualified
0.5 = ekt
ln 0.5 = kt
ln o.5 / t = k
Mensu M.
how did you find the value of 0.5? thank you in advance05/11/22
Still looking for help? Get the right answer, fast.
Get a free answer to a quick problem.
Most questions answered within 4 hours.
OR
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.





Mark M.
Some information from Example 13 is missing.03/04/21