J.R. S. answered • 09/20/19
Ph.D. in Biochemistry--University Professor--Chemistry Tutor
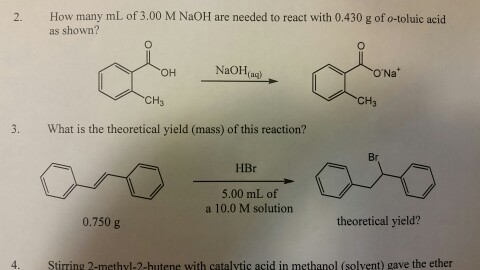
The mole ratio of NaOH to o-toluic acid is 1 : 1 as seen in the balanced equation. The following is the answer to the question in the header, not the answer to the question in the attachment.
0.289 mol o-toluic acid x 1 mol NaOH/mol acid = 0.289 mol NaOH
(x L)(2.50mol/L) = 0.289 mol
x = 0.1156 L = 115.6 mls = 116 mls (to 3 significant figures)