Daniel B. answered • 05/30/21
A retired computer professional to teach math, physics
Let
m = 250 g = 0.25 kg be the mass of the object,
ΔQ = 14 J be the additional heat,
ΔT = 10°C = 10°K be the rise in temperature,
k (to be found) be the specific heat.
The specific heat, k, is the about of heat needed to increase the temperature
of 1 kg of mass by 1°C (= 1°K)
Therefore
ΔQ = kmΔT
From that
k = ΔQ/mΔT
Substituting actual numbers
k = 14J/(0.25kg×10°C) = 5.6 J/kg∙ K


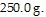 Adding
Adding 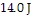 of heat will raise the temperature of the object by
of heat will raise the temperature of the object by 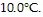 What is the specific heat of the object?
What is the specific heat of the object?


